Description:
Methotrexate (MTX) loaded polymersomes are used to preferentially deliver MTX to sites of ectopic pregnancy
Background
Ectopic pregnancy, abnormal pregnancy implantation at sites other than in the uterine cavity, leads to internal bleeding if not identified and treated in a timely manner, and is a major cause of maternal morbidity and mortality. Accounting for about 1-2% of pregnancies in the United States, ectopic pregnancy is normally treated using a systemic administration of methotrexate (MTX). However, the failure rate of MTX treatment can exceed 10%. The risk factors of such failure are poorly understood, but failure can result in increased dosing and undesirable side effects. There is a need to provide better treatment options for patients with ectopic pregnancies, including improved methods of delivering MTX.
Technology Description
This technology licensing opportunity relates to a novel drug formulation utilizing MTX-loaded polymersomes for the treatment of ectopic pregnancy. Polymersomes are biocompatible, self-assembling vesicles that can encapsulate and deliver drugs efficiently. This technology aims to enhance MTX efficacy by addressing the following challenges:
1. Targeted Delivery: Polymersomes loaded with MTX preferentially accumulate at the ectopic implantation site, improving drug concentration where it matters most.
2. Controlled Release: By using a glutathione‑responsive element, release of MTX occurs upon internalization into placental cells, potentially reducing the need for frequent dosing and minimizing side effects.
3. Single-Dose Treatment: In vivo studies in mice demonstrate that MTX-loaded polymersomes effectively treat ectopic pregnancy with a single administration, potentially eliminating the need for multiple doses.
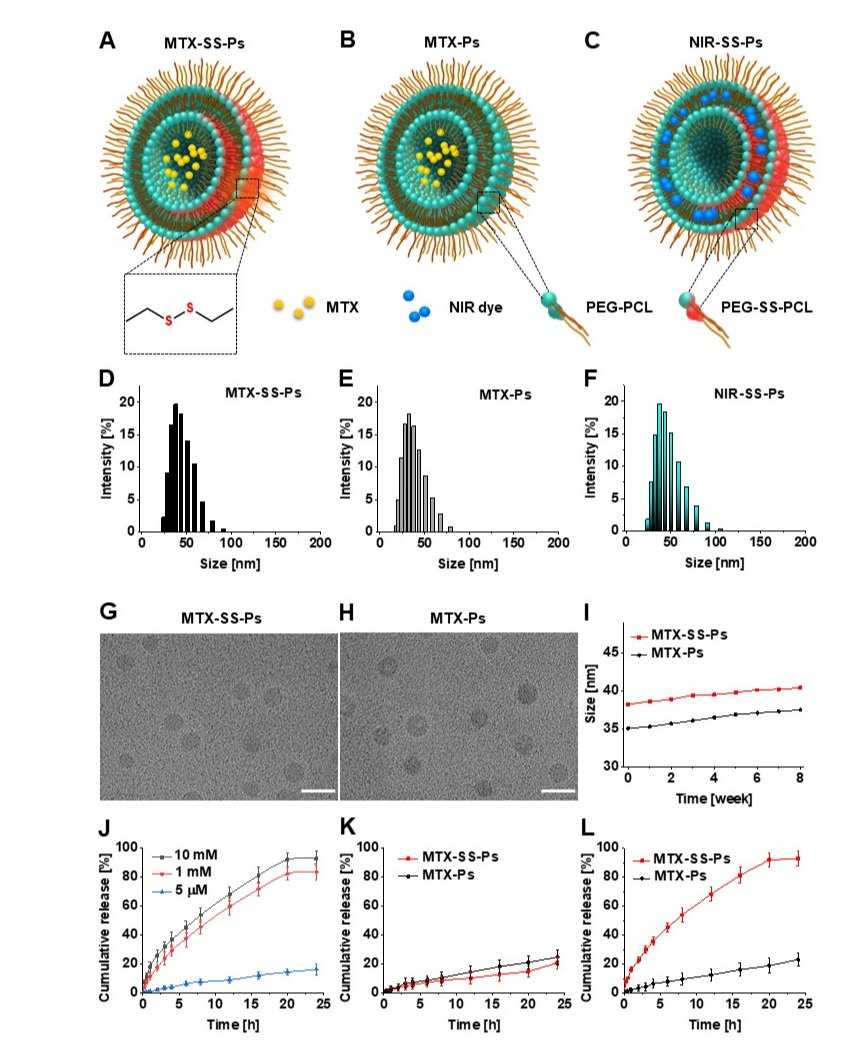
Developed polymersomes and their characterization. Schematic illustration of A) MTX-SS-Ps (PEG-PCL disulfide bond-containing polymersome loaded with MTX), B) MTX-Ps (polymersome lacking disulfide bond and loaded with MTX), and C) NIR-SS-Ps (disulfide bond-containing polymersome loaded with NIR dye (silicon naphthalocyanine). Size distribution measured with dynamic light scattering (DLS) for D) MTX-SS-Ps (38.2 nm ± 0.4, PDI: 0.11, n = 3), E) MTX-Ps (35.5 nm ± 0.3, PDI: 0.10, n = 3), and F) NIR-SS-Ps (39.3 nm ± 0.5, PDI: 0.12, n = 3). Cryo-TEM images of G) MTX-SS-Ps and H) MTX-Ps. I) Stability study via DLS size measurements for MTX-SS-Ps (red) and MTX-Ps (black) over 8 weeks. Drug release profiles of J) MTX-SS-Ps in PBS (pH = 7.4) buffer containing 5 μm, 1 mm, and 10 mm GSH, and K,L) MTX-SS-Ps (red) and MTX-Ps (black) at pH 7.4 K) without GSH and L) with 10 mm GSH.
Further Details: Glutathione-Responsive Methotrexate Polymersomes for Potential Management of Ectopic Pregnancy
Features & Benefits
- MTX‑loaded polymersomes may provide a single dose option for treatment of ectopic pregnancy.
- MTX‑loaded polymersomes may overcome problems associated with the administering of free MTX and rapid clearance of the drug.
- MTX-loaded polymersomes are uniform, highly reproducible, and stable for 8 weeks.
- MTX-loaded polymersomes are synthesized with high MTX encapsulation efficiency.
- The PEG and PCL polymers used are biodegradable and FDA-approved.
- The structure of the polymersomes allows for highly-effective release of MTX to the targeted cells, improving drug delivery and minimizing off-target toxicity.
Applications
Opportunity
Oregon State University is seeking a licensee or development partner. The ideal licensee will have a strong track record of developing early stage technologies into therapeutic treatments and preferably a focus on women's reproductive health and will be motivated and capable of bringing to market an improved MTX therapeutic treatment that improves patient outcomes.
Status
An international PCT application has been filed.